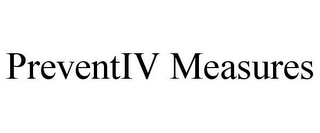
PREVENTIV MEASURES
77860437
Oct 29, 2009
Labels sold as an integral component of pharmaceuticals, namely, alkalizing agents, analgesics and anesthetics, antacids, anticholinergics, anti-infectives, anti-inflammatory agents, anticonvulsants, antiemetics, anxiolytics, blood modifiers, cardiovasculars, diuretics, hormones, diluents, oncolytics, transplantation agents, imaging agents, vascular targeting agents, cancer treatments, and vascular disease treatments; Labels sold as an integral component of pre-filled syringes sold filled with alkalizing agents, labels sold as an integral component of pre-filled syringes sold filled with analgesics and anesthetics, labels sold as an integral component of pre-filled syringes sold filled with antacids, labels sold as an integral component of pre-filled syringes sold filled with anticholinergics, labels sold as an integral component of pre-filled syringes sold filled with anti-infectives, labels sold as an integral component of pre-filled syringes sold filled with anti-inflammatory agents, labels sold as an integral component of pre-filled syringes sold filled with anticonvulsants, labels sold as an integral component of pre-filled syringes sold filled with antiemetics, labels sold as an integral component of pre-filled syringes sold filled with anxiolytics, labels sold as an integral component of pre-filled syringes sold filled with blood modifiers, labels sold as an integral component of pre-filled syringes sold filled with cardiovasculars, labels sold as an integral component of pre-filled syringes sold filled with diuretics, labels sold as an integral component of pre-filled syringes sold filled with hormones, labels sold as an integral component of pre-filled syringes sold filled with diluents, labels sold as an integral component of pre-filled syringes sold filled with oncolytics, labels sold as an integral component of pre-filled syringes sold filled with transplantation agents, labels sold as an integral component of pre-filled syringes sold filled with imaging agents, labels sold as an integral component of pre-filled syringes sold filled with vascular targeting agents, labels sold as an integral component of pre-filled syringes sold filled with cancer treatments, and labels sold as an integral component of pre-filled syringes sold filled with vascular disease treatments; Labels sold as an integral component of injectable drugs and therapeutic solutions for the treatment of cancer and vascular diseases; labels sold as an integral component of injectable drugs and therapeutic solutions for the treatment of pain; labels sold as an integral component of injectable drugs and therapeutic solutions for the treatment of infections; labels sold as an integral component of injectable drugs and therapeutic solutions for the treatment of cardiovascular disease; labels sold as an integral component of injectable drugs and therapeutic solutions for the treatment of hormonal imbalances; labels sold as an integral component of injectable drugs and therapeutic solutions for the treatment of heartburn; labels sold as an integral component of injectable drugs and therapeutic solutions for imaging; Labels sold as an integral part of injectable drugs and therapeutic solutions for the treatment of cancer and vascular diseases sold in kits comprising pre-filled IV bags with devices for intravenous administration, namely, transfer pins, vials, needles, and adaptors; Labels sold as an integral part of injectable drugs and therapeutic solutions for the treatment of pain sold in kits comprising pre-filled IV bags with devices for intravenous administration, namely, transfer pins, vials, needles, and adaptors; Labels sold as an integral part of injectable drugs and therapeutic solutions for the treatment of infections sold in kits comprising pre-filled IV bags with devices for intravenous administration, namely, transfer pins, vials, needles, and adaptors; Labels sold as an integral part of injectable drugs and therapeutic solutions for the treatment of cardiovascular disease sold in kits comprising pre-filled IV bags with devices for intravenous administration, namely, transfer pins, vials, needles, and adaptors; Labels sold as an integral part of injectable drugs and therapeutic solutions for the treatment of hormonal imbalances sold in kits comprising pre-filled IV bags with devices for intravenous administration, namely, transfer pins, vials, needles, and adaptors; Labels sold as an integral part of injectable drugs and therapeutic solutions for the treatment of heartburn sold in kits comprising pre-filled IV bags with devices for intravenous administration, namely, transfer pins, vials, needles, and adaptors; Labels sold as an integral part of injectable drugs and therapeutic solutions for imaging sold in kits comprising pre-filled IV bags with devices for intravenous administration, namely, transfer pins, vials, needles, and adaptors
Pharmaceuticals