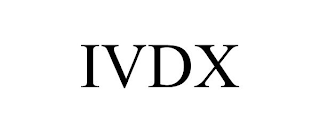
Mark Identification
IVDX
Serial Number
88895735
Filing Date
Apr 30, 2020
Registration Date
Nov 17, 2020
Trademark by
Active Trademark
Classification Information
Regulatory submission management, namely, assisting others in preparing and filing applications for new medical devices with governmental regulatory bodies; Providing consulting services in the field of regulatory submission management to medical companies to assist them with applications for medical device approval
Advertising and BusinessRegulatory compliance consulting in the field of the FDA, FDA guidelines, and medical devices
Personal