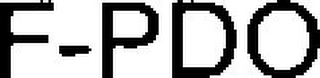
F-PDO
79244654
Jul 17, 2018
Nov 10, 2020
PUBLIC UNIVERSITY CORPORATION FUKUSHIMAMEDICAL UNIVERSITY
Active Trademark
Proteins and protein arrays for use in scientific and medical research; enzymes for scientific and research purposes; reagents for analysis and detection of proteins, other than for medical or for medical or veterinary diagnostic use; cells for scientific, laboratory, or medical research; medium for cell culture or microbial culture, other than for medical or veterinary diagnostic or medical use, namely, cell culture media for scientific and research use; reagents for gene research, other than for medical or veterinary use; inspection reagents for scientific and research purposes consisting of DNA chips and protein chips, other than for medical or for medical or veterinary diagnostic use; blood separating agent in the nature of silicates; albumin from animals or vegetables for scientific and medical research; nucleic acid products, namely, DNA, RNA, oligonucleotides, nucleosides and reagents to conduct sequencing for scientific research purposes other than for medical or for medical or veterinary diagnostic purposes; antibodies, other than for medical or veterinary use, namely, biochemicals in the nature of monoclonal or polyclonal antibodies for in vitro scientific or research use; biological tissue cultures, other than for medical or veterinary purposes; chemical reagents, namely, kits containing chemical reagents for scientific purposes other than for diagnostic, medical or veterinary purposes; chemicals, namely, chemical preparations for scientific purposes, other than for medical or for medical or veterinary diagnostic use; plant growth regulators, namely, substances for regulating plant growth; plant food; higher fatty acids for use in scientific research; test strips, other than for medical purposes, namely, chemically-treated non-medical test strips for calibration of laboratory test equipment; photographic materials, namely, photographic chemicals; vectors, other than for medical or for medical or veterinary diagnostic purposes, namely, nucleic acid products in the nature of molecules for modifying genetic materials in scientific research; plasmid, other than for medical, medical diagnostic or veterinary diagnostic purposes, namely, nucleic acid products in the nature of molecules for modifying genetic materials in scientific research
ChemicalsScientific research, testing and analysis services in the field of life science, pharmaceuticals, genetics, cancer detection and treatment; pharmaceutical testing, inspection, research or development services in the fields of proteins, antibodies, microorganisms and cells; pharmaceutical testing, inspection, research or development in the fields of bacteriology, cytology, antibody technology, chemistry, immunology and gene expression system; pharmaceutical testing, inspection, research or development on cell and tissue culture; testing, inspection, research or development of drugs and biologics for the prevention and treatment of diseases in the fields of autoimmunity, inflammation, oncology, fibrosis and nerve cell degeneration; testing, research, inspection or development of cancer vaccine; pharmaceutical testing, inspection, research or development of clinical trials, drugs, and biologics for the medical treatment of cancer; testing, inspection, research or development on genes, regarding genetic mutation and expression profile, and providing information relating thereto, in the field of genetics and genetic engineering; providing information on testing, inspection or research on DNA sequence, RNA sequence or amino acid sequence of protein in the field of life science; analysis or screening of DNA, RNA or protein in the field of life science for scientific research purposes; testing, inspection or research of pharmaceuticals, chemicals, cosmetics, reagents and foods, and providing scientific advice and information on the foregoing; quality evaluation for others in the field of pharmaceuticals and clinical trials; providing quality assurance services in the field of pharmaceuticals and clinical trials; medical and scientific research in the field of medical treatments for cancers and providing consultation and information on the foregoing; testing and research in the field of biotechnology and providing consultation and information on the foregoing; testing and research in the field of genetics, and providing information on the foregoing; research and consultancy in the field of zoology; testing, inspection or research of pharmaceuticals, cosmetics or foodstuffs; research on building construction or city planning; testing or research on prevention of pollution; testing or research on electricity; testing or research on civil engineering; testing, inspection or research on agriculture, livestock or fisheries; testing the functionality of machines, research on machines and laboratory apparatus and instruments; design for others of machinery and scientific research equipment, including design for others of the structural parts comprising the foregoing machinery and equipment; providing information and advice on computer system design and use of information technology to process information in the medical field, said information and advice provided via computer; computer software design, computer programming, or maintenance of computer software; providing temporary use of on-line, non-downloadable computer programs for medical image analysis via computers; rental of computers; providing temporary use of on-line, non-downloadable computer programs on data networks for the purpose of gathering, managing and reporting on scientific data, research and analysis
Computer and Scientific